"Latest Insights on Executive Summary Viral Vectors-Based Gene Therapy for Non-Human Primates Market Share and Size
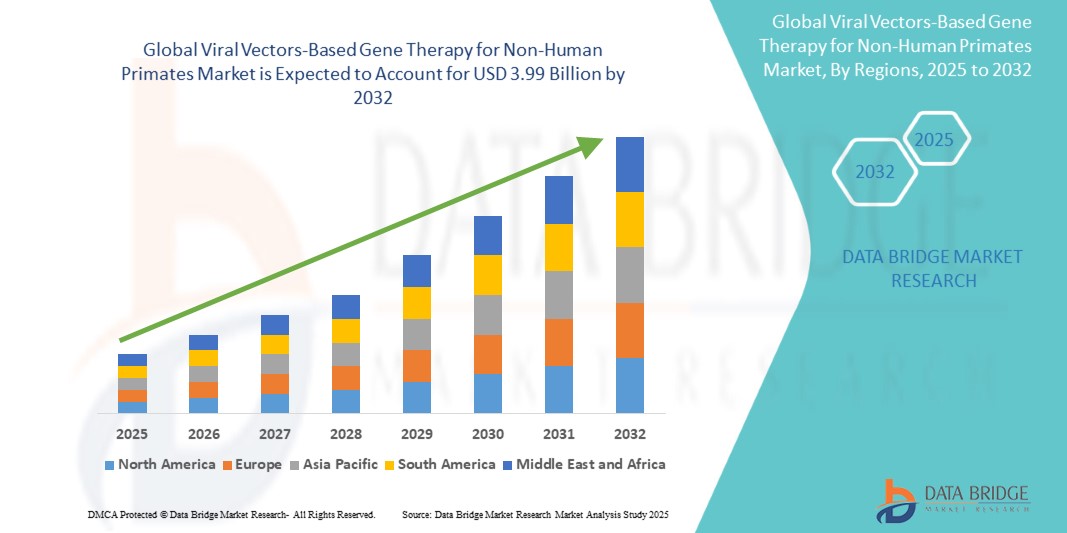
Global viral vectors-based gene therapy for non-human primates market size was valued at USD 1.26 billion in 2024 and is projected to reach USD 3.99 billion by 2032, with a CAGR of 15.40% during the forecast period of 2025 to 2032.
To produce the best market research report, a wide range of objectives is required to be kept in mind. The large scale Viral Vectors-Based Gene Therapy for Non-Human Primates Market report is comprehensive and object-oriented which is structured with the grouping of an admirable industry experience, talent solutions, industry insight and most modern tools and technology. Here, market segmentation is performed in terms of markets covered, geographic scope, years considered for the study, currency and pricing, research methodology, primary interviews with key opinion leaders, DBMR market position grid, DBMR market challenge matrix, secondary sources, and assumptions.
Various parameters taken into consideration in Viral Vectors-Based Gene Therapy for Non-Human Primates Market business report helps businesses for better decision making. This information and market insights help to increase or decrease the production of goods depending on the conditions of demand. It also simplifies management of marketing of goods and services successfully. With the meticulous competitor analysis detailed in this report, businesses can estimate or analyse the strengths and weak points of the competitors which helps create superior business strategies for their own product. A wide-ranging Viral Vectors-Based Gene Therapy for Non-Human Primates Market research report is sure to help grow the business in several ways.
Dive into the future of the Viral Vectors-Based Gene Therapy for Non-Human Primates Market with our comprehensive analysis. Download now:
https://www.databridgemarketresearch.com/reports/global-viral-vectors-based-gene-therapy-for-non-human-primates-market
Viral Vectors-Based Gene Therapy for Non-Human Primates Business Outlook
Segments
- By Vector Type: Retroviral Vectors, Lentiviral Vectors, Adenoviral Vectors, Adeno-Associated Viral Vectors, Herpes Simplex Viral Vectors
- By Indication: Genetic Disorders, Infectious Diseases, Cancer, Others
- By End-User: Research Institutes, Biopharmaceutical Companies, Contract Research Organizations
The global viral vectors-based gene therapy for non-human primates market is segmented based on several key factors. Firstly, by vector type, which includes retroviral vectors, lentiviral vectors, adenoviral vectors, adeno-associated viral vectors, and herpes simplex viral vectors. These vectors play a crucial role in delivering genetic material to target cells for therapeutic purposes. Next, the market is segmented by indication, with major categories being genetic disorders, infectious diseases, cancer, and others. Different types of gene therapies are developed to tackle various medical conditions, leading to a diverse market landscape. Lastly, the market is segmented by end-user, including research institutes, biopharmaceutical companies, and contract research organizations that drive innovation and commercialization in this field.
Market Players
- Novartis AG
- Lonza
- Thermo Fisher Scientific Inc.
- Merck KGaA
- FUJIFILM Diosynth Biotechnologies
- Sirion Biotech GmbH
- Etubics Corporation
- Creative Biogene
- Oxford BioMedica
- Sibiono GeneTech Co. Ltd.
The global viral vectors-based gene therapy for non-human primates market features a competitive landscape with several key players actively contributing to advancements in this field. Companies such as Novartis AG, Lonza, and Thermo Fisher Scientific Inc. are at the forefront of developing innovative gene therapy solutions utilizing viral vectors. Merck KGaA, FUJIFILM Diosynth Biotechnologies, and Sirion Biotech GmbH also play significant roles in providing vector production services and technologies. Other notable market players include Etubics Corporation, Creative Biogene, Oxford BioMedica, and Sibiono GeneTech Co. Ltd., each bringing unique capabilities and expertise to propel the market forward with cutting-edge gene therapy solutions.
The global viral vectors-based gene therapy for non-human primates market is witnessing significant growth and innovation driven by the increasing prevalence of genetic disorders, infectious diseases, and cancer. With advancements in viral vector technology and gene therapy research, the market is poised for further expansion. One key trend in this market is the focus on developing targeted therapies for specific indications, utilizing different types of viral vectors based on their transduction abilities and safety profiles. Companies are investing in research and development efforts to enhance vector design, manufacturing processes, and delivery systems to ensure efficacy and safety in non-human primate models.
Moreover, collaborations and partnerships between key market players and research institutions are driving innovation and accelerating the development of viral vectors-based gene therapies. These strategic alliances enable knowledge sharing, access to advanced technologies, and streamlined regulatory pathways for bringing novel treatments to the market. As the demand for personalized medicine continues to grow, tailoring gene therapy solutions to individual genetic profiles becomes a focal point for market players. This approach not only enhances therapeutic outcomes but also minimizes potential adverse effects, improving patient compliance and treatment success rates.
Another critical aspect shaping the viral vectors-based gene therapy market for non-human primates is the increasing involvement of biopharmaceutical companies and contract research organizations. These entities play a pivotal role in bridging the gap between research and commercialization, facilitating the translation of preclinical findings into clinical applications. By leveraging their expertise in manufacturing, regulatory affairs, and clinical trials management, biopharmaceutical companies and CROs are instrumental in bringing new gene therapy products to market and expanding treatment options for non-human primates and potentially other species.
Furthermore, the regulatory landscape surrounding gene therapy for non-human primates is evolving, with stringent guidelines in place to ensure product safety, efficacy, and ethical considerations. Compliance with regulatory requirements is paramount for market players looking to navigate the complex approval process and bring their viral vectors-based gene therapies to the market successfully. Regulatory agencies worldwide are closely monitoring advancements in gene therapy research, providing guidance and oversight to promote responsible development and commercialization of innovative treatments.
In conclusion, the global viral vectors-based gene therapy market for non-human primates presents lucrative opportunities for market players to innovate, collaborate, and address unmet medical needs in genetic disorders, infectious diseases, and cancer. As research continues to advance and technologies evolve, the potential for transformative gene therapies tailored to individual patient needs grows exponentially. With a strong emphasis on safety, efficacy, and regulatory compliance, the market is poised for sustained growth and breakthroughs in viral vectors-based gene therapy for non-human primates.The global viral vectors-based gene therapy for non-human primates market is witnessing a paradigm shift driven by the accelerating prevalence of genetic disorders, infectious diseases, and cancer. Key market players are focusing on developing targeted therapies tailored to specific indications by leveraging the unique characteristics of various viral vectors. This strategy enhances transduction abilities and safety profiles, paving the way for more effective and safer gene therapy solutions. Collaborations and partnerships between industry leaders and research institutions are fostering innovation and expediting the development of cutting-edge therapies for non-human primates.
Moreover, the active involvement of biopharmaceutical companies and contract research organizations is instrumental in bridging the gap between research and commercialization in the viral vectors-based gene therapy market. These entities bring valuable expertise in manufacturing, regulatory affairs, and clinical trials management, facilitating the translation of preclinical research into clinical applications. By leveraging their capabilities, biopharmaceutical companies and CROs are expanding treatment options for non-human primates and potentially other species, driving further growth in the market.
The regulatory landscape surrounding gene therapy for non-human primates is evolving rapidly, with stringent guidelines in place to ensure product safety, efficacy, and ethical considerations. Market players must comply with these regulations to successfully navigate the complex approval process and bring their gene therapy products to market. Regulatory agencies are closely monitoring advancements in gene therapy research, providing guidance and oversight to promote responsible development and commercialization of innovative treatments. Adhering to regulatory requirements is crucial for market players to establish trust with stakeholders and ensure the successful adoption of viral vectors-based gene therapies.
In conclusion, the global viral vectors-based gene therapy market for non-human primates presents promising opportunities for innovation, collaboration, and addressing unmet medical needs in various disease areas. With a strong emphasis on safety, efficacy, and regulatory compliance, the market is poised for sustained growth and transformative breakthroughs in gene therapy solutions. Continued research advancements and technological innovations will drive the development of personalized gene therapies tailored to individual patient profiles, contributing to improved treatment outcomes and patient care in the non-human primate population and potentially beyond.
Analyze detailed figures on the company’s market share
https://www.databridgemarketresearch.com/reports/global-viral-vectors-based-gene-therapy-for-non-human-primates-market/companies
Viral Vectors-Based Gene Therapy for Non-Human Primates Market – Analyst-Ready Question Batches
- What is the Viral Vectors-Based Gene Therapy for Non-Human Primates Market share of domestic vs international players?
- Which product innovations are most successful?
- What are the logistics challenges in this Viral Vectors-Based Gene Therapy for Non-Human Primates Market industry?
- Which pricing models are most effective?
- What customer acquisition strategies work best?
- How has COVID-19 impacted the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
- What are the main challenges faced by SMEs?
- Which countries are the biggest importers?
- What portion of the Viral Vectors-Based Gene Therapy for Non-Human Primates Market is unorganized?
- How has consumer perception evolved recently?
- Which regions are considered saturated?
- What role does packaging play in consumer choice?
- What loyalty programs are used in this Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
- How is AI being applied in the Viral Vectors-Based Gene Therapy for Non-Human Primates Market?
Browse More Reports:
Europe Food Diagnostics Market
Middle East and Africa Excipients Market
North America Dental Implants Market
Europe Deep Brain Stimulation Systems Market
North America Data Center Busway Market
Europe Critical Communication Market
Germany Critical Communication Market
Europe Biochar Market
Asia-Pacific Amniotic Products Market
Europe Airless Dispenser Market
Europe Aesthetic Dermatology Market
Asia-Pacific Adult Diapers Market
Global Hydrogen Storage Market
Global Medical Spa Market
Global Flame Retardants Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"


